Archive for the ‘Point-of-Care Devices’ Category
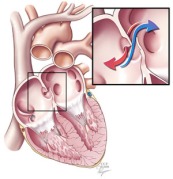
A patent foramen ovale is a defect in the wall between the two sides of the heart that allows the passage of blood and its contents. This course bypasses the lungs. (Courtesy Cleveland Clinic)
One controversial cause of some strokes is a small hole between the two sides of the heart known as a patent foramen ovale. Although rarely symptomatic for patients, this hole allows blood clots that occur in otherwise healthy individuals to bypass the lungs and lodge in critical arteries that serve the brain. In individuals without this defect, these small blood clots would normally lodge in the small vessels of the lungs and typically never lead to a disease state (see note at end for a more complete explanation). Because of the unresolved structural defect, conventional thinking considers these individuals at increased risk for future strokes that remains even after typical stroke prevention strategies.
Kurt Amplatz is an interventional radiologist who has spent his career developing a number of devices to repair these patent foramen ovale and other defects using a catheter-based device that closes the hole with a permanent metal disc. The procedure is similar to cardiac catheterization procedures used for patients with coronary artery disease (e.g., heart attacks).

An Amplatzer occluder deployed on the end of a catheter. (Courtesy St. Jude Medical)
Two recent papers(1,2) in the New England Journal of Medicine report findings from long-term studies designed to demonstrate the expected benefit of using these “Amplatzer” devices versus traditional medical therapies (e.,g., aspirin, Coumadin®, Plavix®). The Journal effectively uses these two studies to demonstrate just how fine a line of improvement may be found with use of the devices and ultimately concludes that true believers and skeptics will likely not be swayed from their opinions by the limited findings of both studies.
What I found most interesting about this recent revival of the debate of catheter-based occlusion devices is the near-zero discussion of the cost component of these devices. The Amplatzer occluder used in these studies was not a one-time quick-fix for these patients but was a supplemental therapy that was often used in conjunction with traditional medical therapies. Although exact pricing is not available, the cost of the device alone adds an additional $3,000-5,000 to the cost of the patient’s care and insurance is usually billed an additional $10,000-$25,000 for the procedure.
With the current evidence, these devices add additional costs and procedural risks to a patient’s care without demonstrating definitive benefit. Addressing the escalating cost-problem in U.S. healthcare starts with regulatory authorities scrutinizing care scenarios such as this one to determine if we are getting value for money in procedural medicine.
Note on blood clots: Venous thromboembolisms are a major cause of morbidity and mortality. However, the specific sequence of events that produces small clots that the human body can easily degrade versus those that cause life-threatening events is poorly understood. Although any blood clot seen in the healthcare setting is typically treated as if it were potentially life-threatening, the general thinking is that small blood clots as in the example given above are somewhat routine in the older population and can resolve spontaneously if not symptomatic.
Editor’s Note: A change in the publication schedule has been made for the month of November. This post below replaces a previous November post on pharmaceutical markets in developing countries that will be re-published in the future.
Disclaimer: Every pregnancy is unique. The below discussion focuses on population-wide assessments of effectiveness and should not be used to make a decision about one’s personal healthcare. Any questions about the issues raised below with regard to the reader’s own pregnancy should be discussed with their obstetrician.
When a pregnant woman arrives to a hospital in labor, she will typically be whisked away to a Labor and Delivery ward. For those familiar with birth, the timing of what follows is highly unpredictable. If in true labor, she will be whisked to the room where she will deliver, and be allowed to progress for the often hours-long wait prior to delivery. After being immediately assessed, most institutions include protocols that have the ward nurses place a continuous fetal heart rate and uterine contraction monitor. The information gathered by the probes is then usually electronically transmitted to a central server for display on any number of desktop monitors and overhead displays scattered throughout the ward. The principal behind this monitoring is for the medical team to be able to readily assess the basic vital signs of labor on any patient from any location in the ward. For example, fetal heart rate decelerations are one recognized sign of fetal distress, and many institutions train staff to immediately respond to such a feature observed on monitoring. A frequent result of “ominous” or “nonreassuring” fetal heart rate patterns is that further observation of the fetal heart rate does not improve the assessment of fetal health and an emergent Caesarean section ensues.

As comforting as continuous fetal monitoring may appear, the devices’ tendency to prematurely warn of fetal distress may actually worsen outcomes for mother and baby. (Photo Courtesy: Medgadget)
Conventional wisdom would suggest that the current widespread distribution of such telemetry monitoring for active labor has saved the lives of countless women and their progeny. Until the twentieth century, pregnancy was one of the most dangerous periods of a woman’s life, and much of this was the result of difficulties during active labor without the ability to safely remove the near-term pregnancy. Even today, Caesarean section – with only minor technical modifications in the ensuing period – remains the standard of care for achieving optimal outcomes when persistent fetal distress is detected during active labor. Logically, one would then think that real-time vitals of the fetus would be the ideal technological assistance for fetal health surveillance during this period of pregnancy.
Although such reasoning appears sound and is routinely used by purveyors of continuous heart rate monitoring, the scientific evidence to date does not support the use of such devices. Although proponents of continuous fetal monitoring have cited a large recent study that suggests otherwise, the best scientific evidence suggests that continuous fetal heart rate monitoring results in the same mortality outcomes for mother and fetus as what can be achieved with the traditional form of fetal health monitoring, or “intermittent auscultation” (a nurse rounding on the mother every 15 minutes and listening for the fetal heart rate with a stethoscope-like device). Worse, not only are mortality outcomes unchanged by an expensive piece of hospital IT infrastructure, but the studies have also shown that the number of Caesarean sections increases with the use of continuous fetal heart rate monitoring. What this constellation of findings suggests is that such monitoring does not improve health outcomes but also has the unfortunate disadvantage of “sounding the alarm” too early. In other words, continuous monitoring appears to be picking up fetal distress that is transient that may be more accurately described as “fetal discomfort.” These findings suggest that if such cases were left alone, the distress would often resolve on its own without any lasting effect on health outcomes for mother or baby.
To summarize, the amassed evidence to date suggests that continuous fetal monitoring does not improve newborn mortality and increases the number of C-sections performed when it is used. Why then is it the standard of care across much of the U.S.? I’ve put exactly this question to a number of my mentors and colleagues, and the answer is invariably the same. Evidence aside, no jury in the United States is going to be kind to the obstetrician who goes against the tide of medical practice to adhere to what “stuffy” academics suggest is a more effective treatment strategy. That a minority of institutions in the U.S. even still allow practitioners to use intermittent auscultation is testament to the American College of Obstetrics and Gynecology’s measured acceptance of both forms of fetal monitoring.
What troubles me the most about the state of fetal monitoring today is the potentially massive quality transformation that exists and the limited work being done to explore this issue further. Labor is one of the most common conditions requiring hospitalization and the question of how it is monitored affects every single one of those admissions. Definitive research is needed to settle this debate. Because such research may very well prove the futility of a profitable medical technology, it is likely that professional advocacy is the only way such work will get the necessary funding to conduct a large randomized trial. Without it, we will continue to perform a practice that is contributing to what may be thousands of unnecessary surgeries a year and a considerable cost burden to our healthcare system.
Disclosure: I have no financial or professional interests related to continuous fetal heart rate monitoring or other matters discussed above.
I recently came across FoetoH, a fetal heart rate monitoring device that has been developed at the University of Oxford. Unlike other forms of fetal health monitors, FoetoH is designed to be used by laypeople and in a real-time manner. Rather than giving health information output in complex jargon or graphs, the device provides a stoplight-style assessment (green, yellow, red) of a developing fetus’ current health. The scientific breakthrough was developing an exercise belt-like device (think exercise heart rate monitors) that a mother-to-be wears all the time that communicates with a handheld unit (or iPhone app) which facilitates data storage and interpretation.
The idea of FoetoH is attractive because of its synthesis of the latest technology trends (i.e., mobile-based health applications, user-oriented design) and advanced health monitoring devices. The marketing materials of FoetoH are excellent and describe this device as potential breakthrough to help address the more than 2 million stillborn babies born each year around the world. The basis of this claim is that mothers who know their pregnancy is in trouble (indicated by a “yellow” or “red light” on the device) could receive emergent medical care to improve fetal outcomes.
Unfortunately, such a simplified product and health solution obscure some major logical flaws in their existing argument. For FoetoH to contibrute to a reduction in worldwide stillbirths, the device needs to prove itself to be more than just effective at measuring fetal heart rates. FoetoH’s founders need to be able to demonstrate that identifying changes in fetal heart rates is an effective way of identifying AND preventing still births. Why do I raise this issue? The limited data available on still births demonstrates that the majority of still births are due to genetic and environmental insults that go well beyond impaired cardiovascular support of the fetus. Many of these stillbirths are due to unknown genetic causes, infectious disease, or severe malnourishment, and fetal distress (erratic fetal heart rates) is an end-stage sign of imminent still birth. In these cases, last-minute emergency care would have virtually no chance of preventing “fetal demise” (technical term for still birth). It is also unclear that FoetoH’s real-time monitoring is any more effective than current guidelines for antenatal care which include regular physician visits and routine ultrasound scans at pregnancy milestones.
Moreover, it is unlikely that most mothers at risk for stillbirth would be able to gain access to the FoetoH device. Its currently reported cost of manufacturing is approximately $80. A public sector price is likely at least twice as expensive with a market price even more. Given that the vast majority of stillbirths occur in impoverished women from developing countries, the target population who could potentially benefit from such a device would be unlikely to be able to afford it. Even if such devices were provided free of charge to high-risk mothers, the limited benefit of using the device I raised in the prior paragraph would likely outweigh the high cost to health systems.
These issues are not lost on healthcare device makers familiar with the product. At Oxford’s recent TATA Idea Idol business plan pitch competition – where FoetoH was a finalist – judge Will Chadwick of TATA Interactive Systems noted that the only realistic market for FoetoH were overly concerned mothers from the industrialized world who were willing to pay for a device that provided peace of mind rather than a clear-cut medical benefit over existing practices.
In fairness to FoetoH, its TATA Idea Idol team went on to win this year’s competition despite Chadwick’s misgivings (so someone clearly thinks FoetoH has something going for it). In the end, the science and potential commercial market for the device were convincing enough to beat out a number of strong competitors. FoetoH is a useful reminder for clinicians. Sound science and commercial availability do not make good medicine. Healthcare providers have to always maintain a critical eye and question new healthcare good and services to ensure that they are consistent with the individual provider’s aims and means of care as well.
Jerry Sanders, one of the medical device industry’s top entrepreneurs, visited Saïd Business School last month for a series of masterclasses on his role in the startup industry. Sanders’ firm San Francisco Science is legendary for its ability to identify promising innovatons that big medical device companies will pay handsomely for. Given the firm’s success, it was quite a shock when Sanders acknowledged that SF Science’s activities were being gradually run down because of what he believed to be the decreasing attractiveness of the market for independent medical device developers. To that effect, Sanders has reduced the number of deals he runs per year from greater than twenty to two active deals currently and only takes on new projects that meet an unusually high level of return for the risk involved.
To summarize Sanders’ argument, independent medical device firms — often only inventor, engineer, financial-whiz, and a few support staff — have been disproportionately impacted by the FDA’s increasing conservatism in the approval of new drugs and medical devices. Following the Vioxx recall, regulatory approval has become both more difficult and more expensive. This constellation of problems has made it near- impossible for a small medical device firm with perhaps 2-3 early-stage products to actually become commercially successful on its own. Essentially, the only option available to bring a medical device to market is to quickly identify a consolidated medical device company (e.g., Johnson & Johnson, Covidien) who is interested in adding a particular innovation into an existing product platform. This situation puts independent medical device innovators into a difficult strategic position. Rarely will more than one or two of the diversified device companies have interest in a particular innovation, but the inventor is often looking for a quick outcome that either funds him or her to progress with further development or exit and move on to something else. A desperate seller and a large, powerful buyer allows for the latter to capture much of the value generated from the deal and leaves the entrepreneur with limited upside.

Does Signostics even have a chance? (Courtesy: Signostics)
To use a real-world example from an earlier post, has the GE VScan device already beaten other handheld ultrasound competitors like Signostic’s Signos Personal Ultrasound because whatever incremental feature benefit Signostics may have will never allow the firm to compete with such an established player in a highly regulated industry? According to some of the leading lights of this industry, the only option for many of these smaller firms is early exit.
What explains Sanders’ complete exit from this industry is that the difficulties he spoke of have little or no chance of disappearing soon. The contentious state of American politics makes ambitious change at the FDA highly unlikely in the near future (although future rumblings do exist). With the increasing role of corporate influence on future regulation, it is likely that any reforms to the FDA device approval process will likely benefit large diversified device firms as much if not more than they do the smaller independent device makers. During a masterclass, Sanders noted that contrary to conventional wisdom “Big Business” (defined here as large consolidated device makers) often welcomes increased regulatory oversight because such hurdles disproportionately hurt small, new entrants to their established industries.
While incremental improvements to existing medical devices will continue to be churned out by the “majors,” the current business and regulatory environment of the medical device industry make the emergence of new, disruptive innovations far less likely.
Why an amazing product can’t find a market

The Gold Standard: GE Vivid 7 (Courtesy GE Healthcare)
Medical ultrasound is considered one of the most effective imaging modalities for cheap, reliable diagnosis of many disease states from heart disease to infected abscesses. Since the technology’s inception, device manufacturers have been steadily miniaturizing components so that what used to be a desk-sized machine (The GE Vivid7 in the picture to the left is a modern version of these behemoths) has now been succeeded by a laptop bolted to a small rolling cart with a number of ultrasound probes hanging off the side.
In late 2009, GE Healthcare released its VScan (see picture on right) handheld ultrasound device to the American market. The VScan device was most notable for its size — only slightly bulkier than a classic clam-shell mobile phone — and a price that was 80% less than traditional machines. Although VScan’s introduction was accompanied by a number of competing devices from other major industry competitors (e.g., Siemens’ ACUSON P10, Signostics’ Signos) as well as enterprising start-ups, the VScan has generally been considered to be the superior product in the pocket portable product segment because it functions near-identically to larger laptop-based portables but in a smaller form-factor. (Still not quite following? See a short introductory video by GE here.)

GE VScan (Courtesy GE Healthcare)
The VScan’s entry should have been heralded by doctors in a variety of clinical settings. A device exists that a clinician can carry in his or her pocket that with relatively little additional training can be used to detect traumatic injuries, major vessel disease, heart failure, and other maladies all without the need for any further appointments (in the outpatient setting) or bulky equipment often confined to a poorly accessible imaging suite. Unfortunately, the VScan and its competitors have had little adoption in the years since their introduction.
The lack of interest in the VScan illustrates how the current reimbursement system of the American healthcare system has direct effects on how patient care is provided. An example of these skewed incentives can be readily seen in one of ultrasound’s largest markets – outpatient cardiac ultrasound. Without VScan, a cardiologist orders a comprehensive ultrasound study that typically requires a second visit and an advanced cart-based ultrasound machine. Each study performed earns the cardiologist a professional and technical fees exceeding $1,500. In contrast, current reimbursement policies (typically set by Medicare and then voluntarily adopted by private insurance companies) do not cover ultrasound procedures performed with handheld devices so physicians are unable to charge for this service. Thus, the $7,000 cost of each handheld ultrasound machine is not going to be recouped through additional procedural charges.
The only revenue stream from use of the VScan product is any potential efficiency gains met by being able to rule-out serious underlying disease quickly and thereby see more patients in the same amount of time. While such a hidden benefit may be difficult to convey to a private practitioner or a standalone healthcare system, integrated healthcare systems (e.g., U.S. Veterans Affairs Hospital Systems, National Health Service) stand to benefit more from lowering the number of unnecessary comprehensive ultrasound exams. It should be no surprise then that integrated healthcare systems in Europe have been some of the few markets where handheld ultrasound devices are regularly used and clinically studied.
Personally, I’m not convinced by proponents of the VScan that such devices will soon replace the conventional stethoscope. The price and relative ease of use the latter are so superior that any real competition between the two is still a decade or more away. Such a claim is analogous to saying the typewriter would make the pencil obsolete. What is far more likely is growing demand for VScan and its competitors as the American healthcare system wakes up to cost-effective care. A greater focus on comparative cost-effectiveness will lead to reimbursement policies rewarding physicians for at least the limited use of such handheld devices versus comprehensive ultrasound studies. As GE’s marketing efforts in the ultrasound industry demonstrate, the real success of such a product can only be evaluated as a component of a greater imaging ecosystem. For a GE, the real value of such a device is that it allows the company to offer an end of the spectrum poorly matched by any of its competitors.
Conflict of Interest Disclosure: I have never received funding from and have no financial stake in GEHealthcare or its subsidaries. In 2010, GEHealthcare loaned a LOGIQ i portable ultrasound machine to a humanitarian surgical trip I led to Hinche, Haiti.





